Academia can be very competitive. Getting a job is competitive, at least tens of very good people will apply for a job at a reputable university. Fellowships that are stepping stones to academic positions have success rates of around 10% — so 90% of applicants fail. And once you are an academic, it does not ease off. You are under pressure to get grants and again, around 90% of grant applications fail. And government policy has tended to push for more and more competition, between universities and between academics. But, faced with the threat of COVID-19, cooperation is breaking out.
Dissolving chalk and the Schmidt number
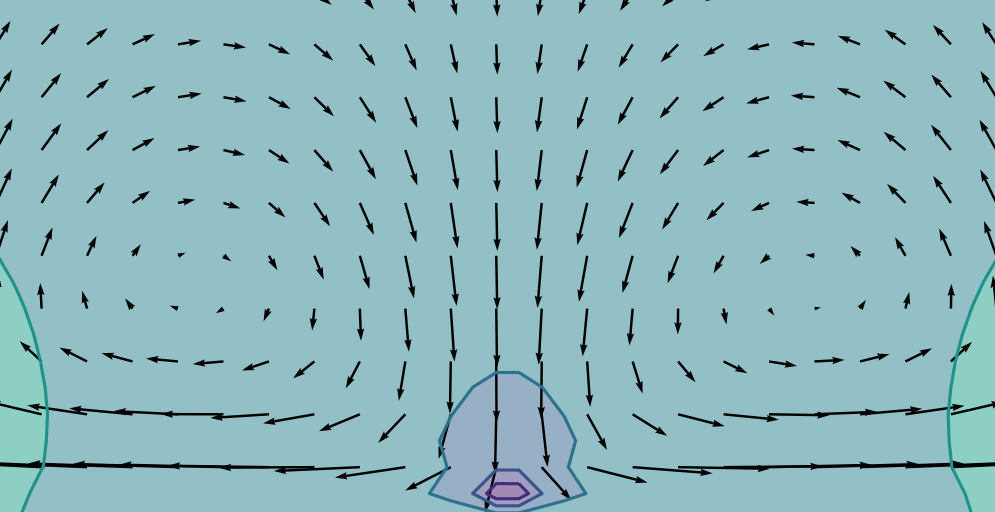 Eight years ago a group at Penn State University published a paper I like a lot. What they did is simple: They just dropped a very small (about a hundreth of a millimetre across) piece of chalk (calcium carbonate) into water, and watched how small charged particles behaved near the dissolving piece of chalk. Chalk is not very soluble which is why if you stick a big piece in water only a small fraction will dissolve, but they were using tiny pieces which dissolve in water over, I think, about an hour or so.
Eight years ago a group at Penn State University published a paper I like a lot. What they did is simple: They just dropped a very small (about a hundreth of a millimetre across) piece of chalk (calcium carbonate) into water, and watched how small charged particles behaved near the dissolving piece of chalk. Chalk is not very soluble which is why if you stick a big piece in water only a small fraction will dissolve, but they were using tiny pieces which dissolve in water over, I think, about an hour or so.
Worrying about inhaling more than the smell of coffee
Way back in the innocent days of 2018, I had a very mild tussle with that part of the BBC that runs BBC Bitesize, which is the part of the BBC’s website dedicated to providing educational resources for school children (original blog post here). The BBC’s webpage originally claimed that the smell of coffee could travel across a (then open) coffee shop, via diffusion. This is not correct, it is carried by air currents, and the BBC did update the webpage.
Every university can be the best!
 There are many university league tables out there: The Guardian’s, The Complete University Guide’s, The Times Higher Education’s, The Student Hut’s, …. and now there is even a league table where universities from my native Wales take seven of the top ten positions. Congratulations to Aberystwyth on topping that table! It occurred to me that I might as well take this proliferation to its logical conclusion and make league tables so that every university can top at least one table. So without further ado, here is a university league table with Surrey as number one university:
There are many university league tables out there: The Guardian’s, The Complete University Guide’s, The Times Higher Education’s, The Student Hut’s, …. and now there is even a league table where universities from my native Wales take seven of the top ten positions. Congratulations to Aberystwyth on topping that table! It occurred to me that I might as well take this proliferation to its logical conclusion and make league tables so that every university can top at least one table. So without further ado, here is a university league table with Surrey as number one university:
rank University 1 Surrey 2 Oxford 3 Northumbria 4 Edinburgh 5 Glasgow 6 Liverpool John Moores 7 Aston 8 St Andrews 9 Bath 10 Durham
Why small particles can’t get through masks
 Masks are in the news at the moment. The basic idea of a mask is simple, it filters out some of the nasty particles from the air. The hope then is that if a droplet containing virus particles gets sucked into or blown out through the mask, it will be trapped by the mask and go no further. How masks filter out the bigger droplets is easy to understand. Masks are basically made from meshes of fibres that are very roughly around ten micrometres or so thick. So when the user breathes in or out, the air is forced through holes in this mesh of fibres. Some of these holes may be only around a micrometre or a few micrometres across, and of course any droplet bigger than the hole will get trapped and so not get through.
Masks are in the news at the moment. The basic idea of a mask is simple, it filters out some of the nasty particles from the air. The hope then is that if a droplet containing virus particles gets sucked into or blown out through the mask, it will be trapped by the mask and go no further. How masks filter out the bigger droplets is easy to understand. Masks are basically made from meshes of fibres that are very roughly around ten micrometres or so thick. So when the user breathes in or out, the air is forced through holes in this mesh of fibres. Some of these holes may be only around a micrometre or a few micrometres across, and of course any droplet bigger than the hole will get trapped and so not get through.
Scaling down someone else’s idea
 When you have as few genuinely original ideas as I do, one way to make progress is to borrow (with appropriate attribution) other people’s ideas. I have been wondering about how a virus such as the corona virus (shown on the left as the knobbly object*) gets through the mucus (pale blue) that lines the inside of nose and throat, to attack the cells (pink) underneath this mucus. Viruses need to get inside our cells to take them over and allow the virus to reproduce.
When you have as few genuinely original ideas as I do, one way to make progress is to borrow (with appropriate attribution) other people’s ideas. I have been wondering about how a virus such as the corona virus (shown on the left as the knobbly object*) gets through the mucus (pale blue) that lines the inside of nose and throat, to attack the cells (pink) underneath this mucus. Viruses need to get inside our cells to take them over and allow the virus to reproduce.
One of the functions of mucus may be to trap or somehow to provide a barrier to the viruses. After some Googling it occurs to me that this problem of blocking movement of a virus is more-or-less the same as the problem water companies have, although the scales are very different.
Will the corona virus improve university assessments (in the long term)?
A sixth form teacher, Niamh Sweeney has an interesting and passionate call for schools and colleges to use the impetus provided by the sudden corona-virus-imposed changes, for good. It is in today’s The Guardian. I teach the products of these schools. I get frustrated by some attitudes I see in students that they may have learnt in the “testing hamster wheel” she refers to. So I hope that the impetus does get used for good in the UK’s schools and colleges.
Roomy compartments
The movie above shows liquid droplets (green) formed by a protein. It is from work by Langdon and coworkers. Over the last few years, here has been an explosion of interest in proteins that phase separate inside living cells to form droplets like this. It appears to be quite common, the cells of our bodies seem to full of droplets that form and dissolve all the time.
End of an era
One of the giants of twentieth century physics, Philip Anderson, died at the weekend, aged 96. He retired from Bell labs in the 1980s and from Princeton University maybe 20 years later, but a couple of years ago we were briefly at the same workshop at Princeton. Bell labs was where the solid state transistor — at the heart of all modern computers — was invented.
Viruses in motion
 A lot of my research looks at how to move particles around, particles about 100 nanometres across. For example when we made stratified coatings we did this with a mixture of particles of sizes 60 and 400 nanometres. The corona virus (see above) is about 100 nanometres across. Viruses are barely alive. They have no metabolism themselves, no more than the inert colloidal particles my colleagues and I study. The virus above has the genetic material in the centre, surrounded by a protein coat. The red things are its spike proteins, that help it enter and infect a cell. The colours above are false, I think it is reconstructed from electron (not light) microscopy.
A lot of my research looks at how to move particles around, particles about 100 nanometres across. For example when we made stratified coatings we did this with a mixture of particles of sizes 60 and 400 nanometres. The corona virus (see above) is about 100 nanometres across. Viruses are barely alive. They have no metabolism themselves, no more than the inert colloidal particles my colleagues and I study. The virus above has the genetic material in the centre, surrounded by a protein coat. The red things are its spike proteins, that help it enter and infect a cell. The colours above are false, I think it is reconstructed from electron (not light) microscopy.